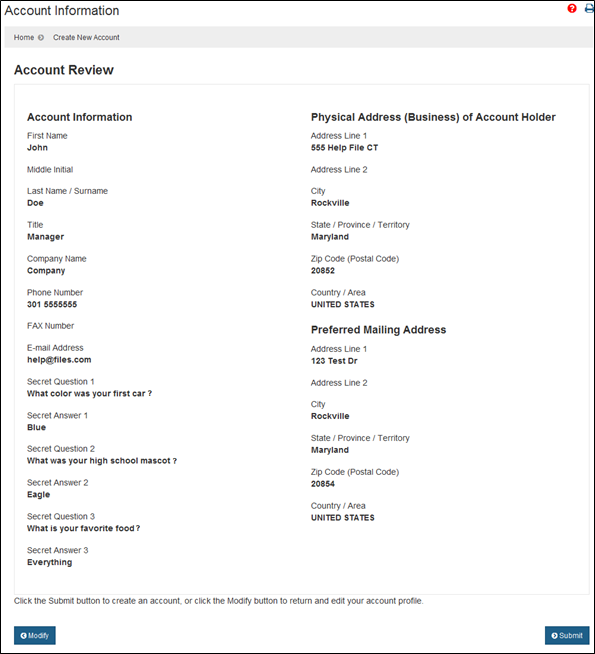
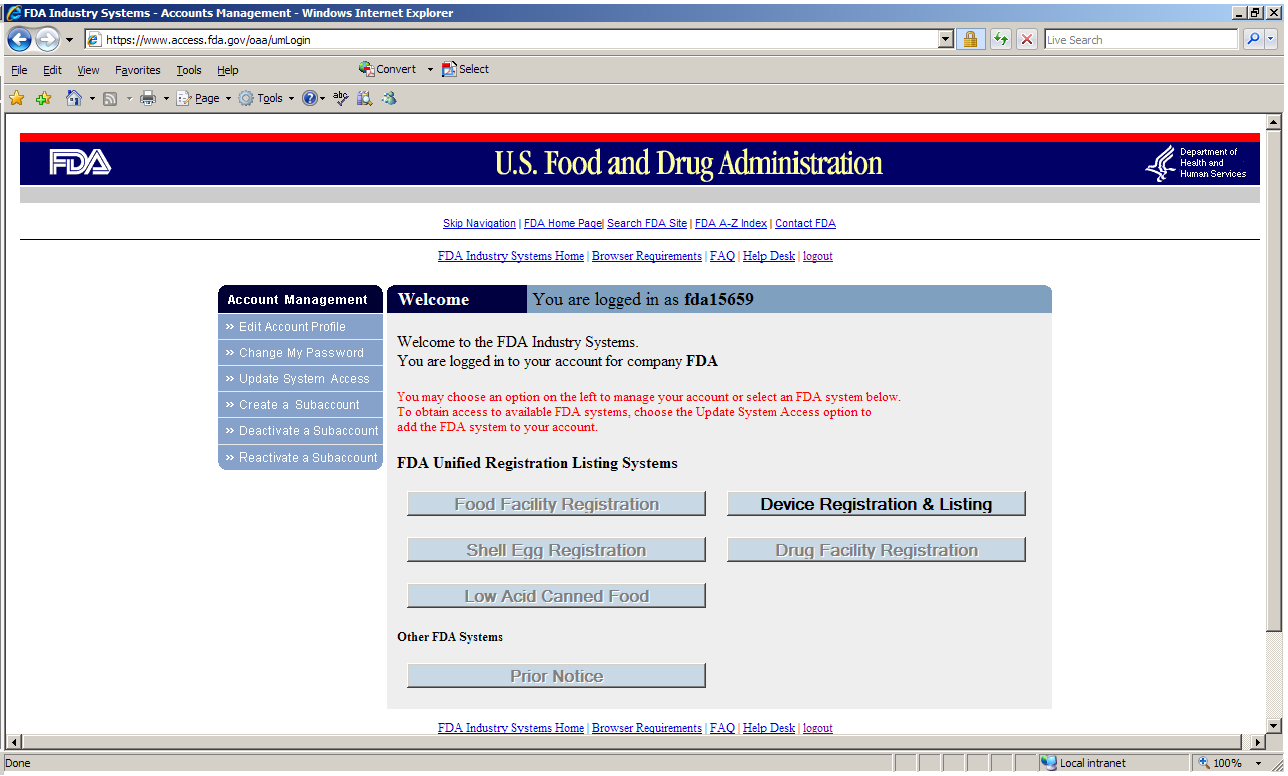
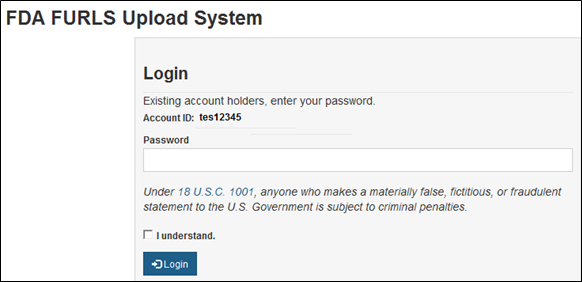
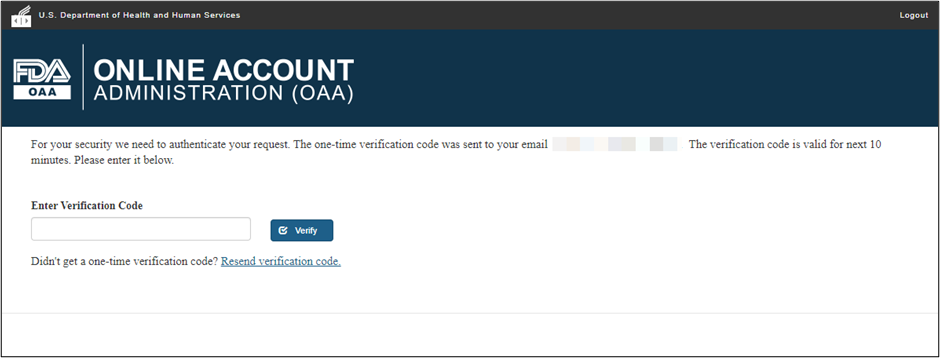
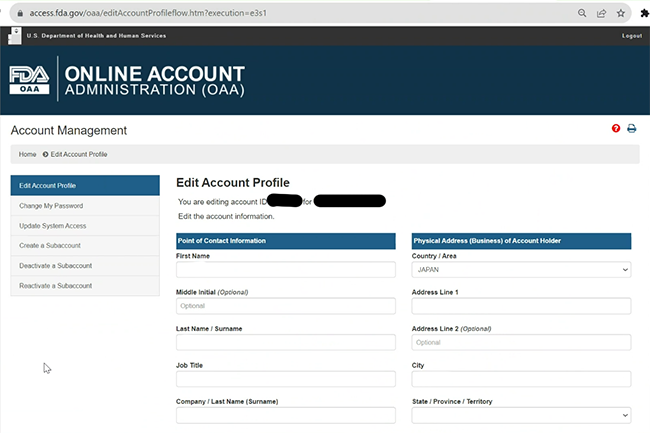
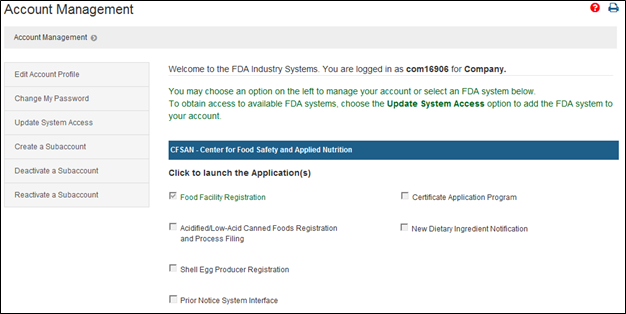
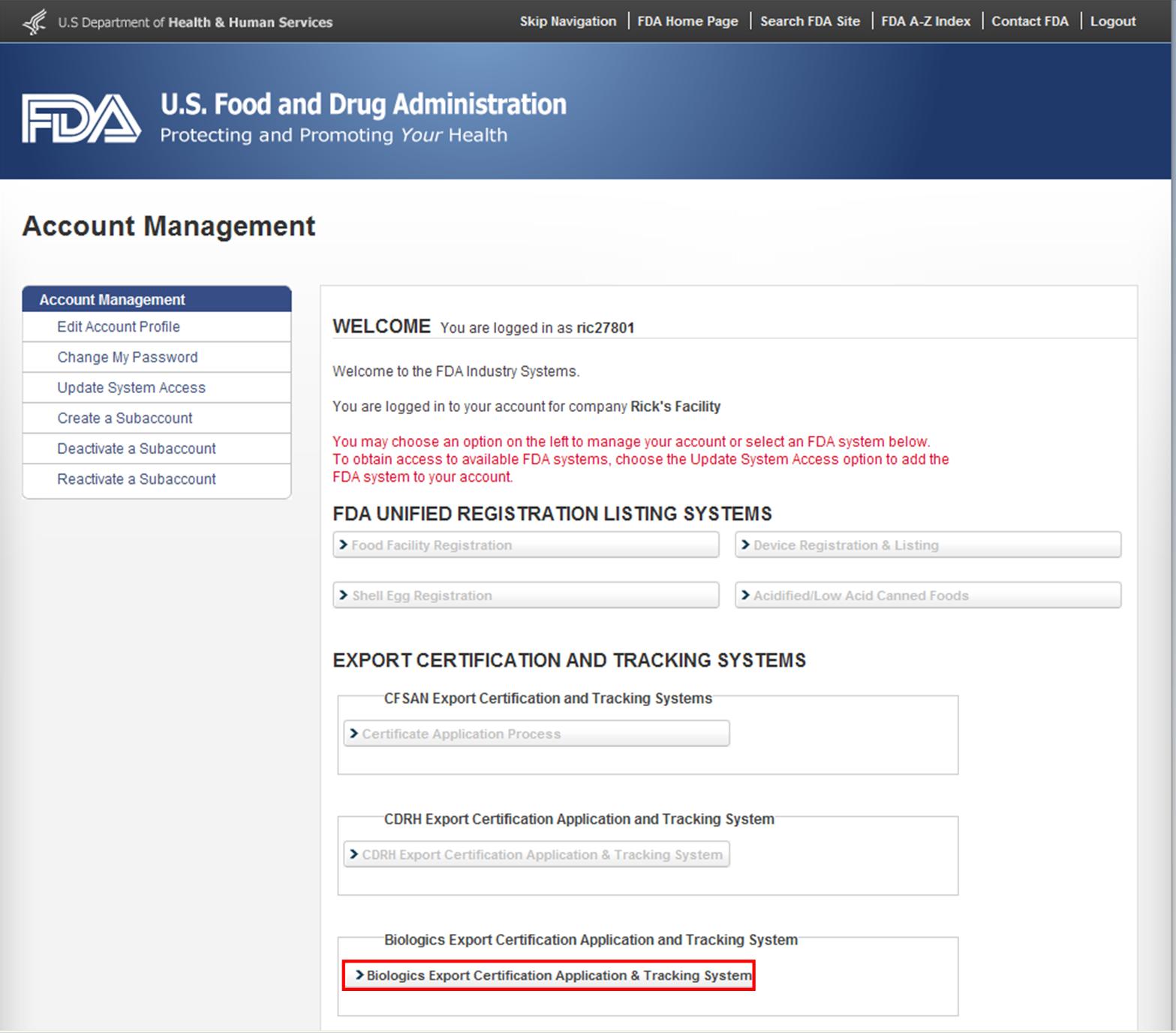
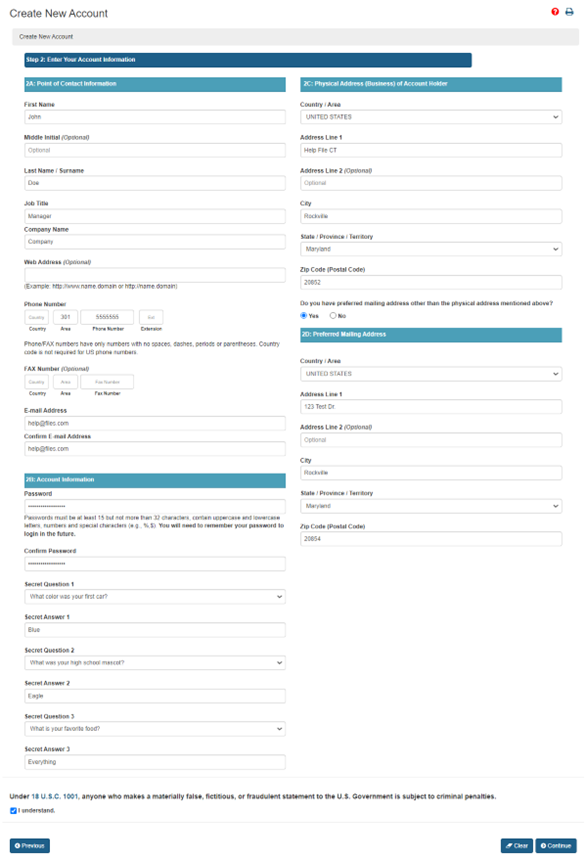
Navigating Regulatory Wasters: A Comparative Dive into FDA Audits vs EU Audits in the Medical Device Industry

New | Cybersecurity in Medical Devices | Quality System Considerations and Content of Premarket Submissions

Hairise FDA Approved Industry Modular Belt Conveyor System - China Chain Conveyor, Spiral Conveyor | Made-in-China.com

FDA Drug Information on X: "FDA issued a final guidance that provides industry, investigators and others recommendations on the use of digital health technologies (DHTs) to acquire data remotely from participants in

US FDA Plans to Issue Electronic Export Documents for Medical Device Industry From January 2024 | Operon Strategist

Smoore Tech Invited to Address FDA Industry Meeting - Smoore International (06969) We are the world's leading atomization technology solution provider

The FDA and Worldwide Quality System Requirements Guidebook for Medical Devices: 9780873893770: Medicine & Health Science Books @ Amazon.com
28 avril 2023 : Microbiome Post - MaaT Pharma Announces U.S. FDA Lifts Clinical Hold on Phase 3 Investigational New Drug Application for MaaT013 in Patients with Acute Graft-versus-Host Disease (English only) - MaaT Pharma